Using an automated endpoint adjudication software such as eAdjudication® helps streamline the whole process while guaranteeing a charter-compliant workflow and timely adjudication of the events. Let’s review the software-integrated six-step process that supports endpoint adjudication performance.
As a cloud-based endpoint adjudication software, eAdjudication® provides independent interfaces for investigational sites, Endpoint Adjudication Committee (EAC) members and Adjudication Central Staff (Endpoint Office) who, through the platform, exchange medical records, redact personal information, assemble and submit dossiers, manage queries, review data packages, perform assessments and resolve any disagreement. All these operations are carefully planned in the platform during the configuration stage.
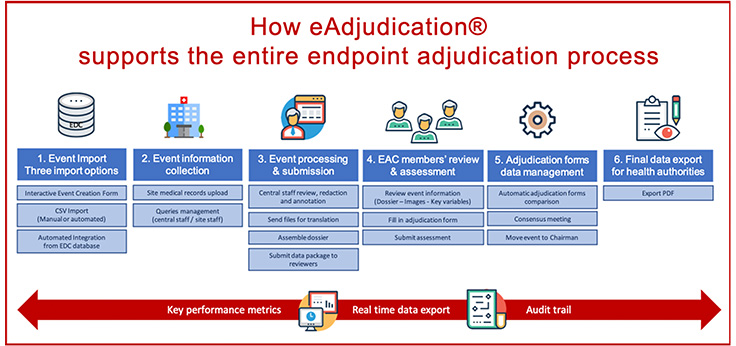
Step 1. Event import into the platform
When an event takes place, the first step is to import the event data from the EDC database. This can be done manually but should be automated if a large number of events is anticipated.
Step 2. Event information collection
The investigational site staff is prompted to upload the event-related medical records in the platform. If a document is incorrect or missing, the central staff communicates to the site by sending a query through the platform.
Step 3. Event information processing and submission
The central staff also uses the platform to send the files to the translation provider if translation is needed, to redact personal information, and to write notes for the reviewers. Once all the documents are ready, the central staff assemble them into the Event Dossier using the built-in tool. The event data package is then sent to the reviewers.
Step 4. EAC members review and assessment
At this stage, the reviewers review the event information (subject profile, event dossier, key variables, event images…), in an independent and blinded way, and provide their assessments using the interactive adjudication form inside eAdjudication®.
Step 5. Adjudication forms data management
When all the assessment forms have been submitted, the system compares them. If the results match, the adjudication for that event is marked closed. If the results don’t match, the event is considered in “disagreement” and eAdjudication® supports the setting up of a consensus meeting. The platform also offers the possibility to present such events to the Chairman, whose assessment will be considered final.
Step 6. Final data export for health authorities
An important benefit of using an automated endpoint adjudication software is its ability to store and track all the operations performed in the system. The platform holds information useful for the study conduct (real time data export, study performance metrics) and for final submission to health authorities.
All the data and records necessary for reviewers’ assessments of the endpoints as well as timelines and workflows must be defined and described in the endpoint adjudication charter during the protocol development phase. The platform can then be configured to exactly match the endpoint adjudication charter.
DOWNLOAD NOW THE FREE ENDPOINT ADJUDICATION HANDBOOK
The Complete Manual / Reference Book (34 pages) with all the topics related to the Independent Endpoint Adjudication Committees Management








