Several regulatory guidelines recommend that assessments of endpoints supporting drug approval should be verifiable by applicants and the regulatory agencies to minimize the potential for bias. This becomes especially critical when assessments are not based on measurable data but are derived from the interpretation of measurements, when they require the application of complex endpoint assessments, or when a study cannot be blinded. To make such interpretation more robust, a verification of (subjective) assessments by an independent panel of experts is frequently utilized.
ABSTRACT
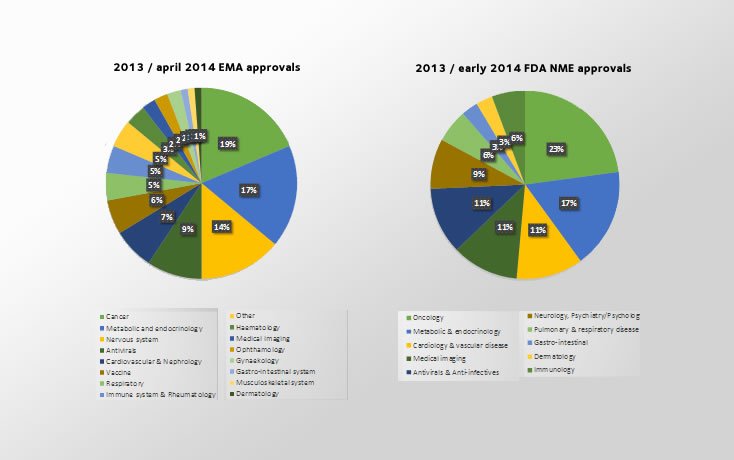
The objective of this paper was to analyze how often adjudicated methods across efficacy and safety assessments were used in drug approvals in the European Union and United States in 2013 and early 2014.
Methods:
A total of 35 new molecular entities (NMEs) approved by the US Food and Drug Administration (FDA) and 88 European Medicines Agency (EMA) approvals in Europe were included in this analysis.
Results:
[...]
Conclusion:
[...]
DOWNLOAD THE FULL-TEXT ARTICLE and FIGURES