The Endpoint Adjudication in Clinical Trials Linkedin Group and Ethical Clinical designed and executed an industry survey that evaluates the impact of technology utilization in clinical endpoint adjudication.
Clinical trial endpoints adjudication is relatively unexplored; several experts argue that the field of endpoint adjudication lacks clarity, standardization, and that there are no existing definitive regulatory policies on the process.
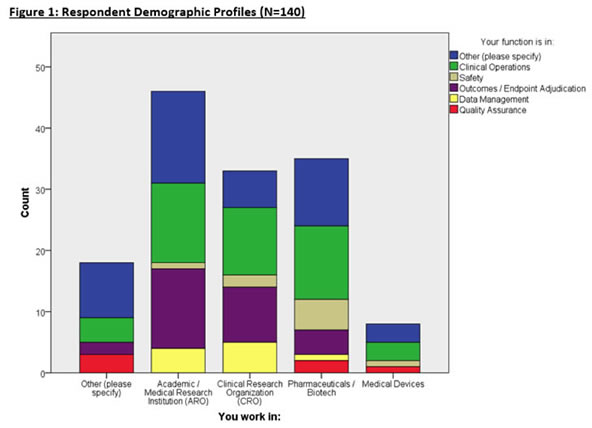
Respondent Demographics
140 eligible respondents completed the survey from a variety of organizations and functions (Figure 1). Most respondents are affiliated with Academic Research Organizations (AROs), CROs, and Pharmaceutical/Biotech enterprises with many respondents working in Clinical Operations, and Endpoint Adjudication. Other respondents comprised of consulting, computing industry, and non-profit organizations.
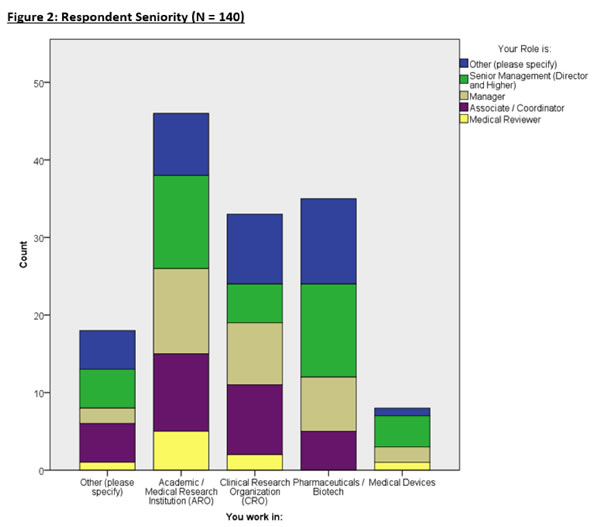
Figure 2 breaks down the seniority of each respondent in specific organizations.
[... ] see below for full-text download.
Endpoint Adjudication Survey Organization
[... ] see below for full-text download.
Challenges with Endpoint Adjudication Software Use
[... ] see below for full-text download.
Cultural Adoption Challenges
[... ] see below for full-text download.
Process Challenges
[... ] see below for full-text download.
Expert Opinion
Endpoint adjudication experts are in accord with these findings.
Dr. Beat Widler of Widler and Schiemann, Ltd., said, “these findings make sense, and I would not wait for regulators or even ICH to provide guidance on the adjudication process. I would invite interested stakeholders to convene and develop best practices through non-profit organizations such as ACRES. There is successful precedent to this approach: a DIA group convened to develop a TMF template, which is now widely used across the industry.”
Dott. Mimmo Garibbo of Ethical Clinical added, “We can all agree that using a dedicated Software Solution can improve efficiency. However, it is also important to note that the Software Solution must be validated to meet federal and quality assurance requirements. Additionally, we need to consider that Software Solutions must be user-friendly and flexible in order for Endpoint Adjudication staff to realize improvements in efficiency without adding new software-related assignments and subsequent work burden."
DOWNLOAD THE FULL-TEXT ARTICLE AND FIGURES




